Friend,
Since you are a person that understands science and math. The proof is simple and I'll be direct.
First we need to understand that any green house effect is not just
about CO2; but the atmosphere as a whole. It's the overall coefficient
of that system that determines any greenhouse effect that might be
present. This gives rise to the following calculations based on known
coefficients and hard science not conjecture.
There exists an equation that says the temperature of that
system "t" can be predicted using the
coefficient of that system. Where t= f(x). The "x" is the overall
Coefficients of that system. Since this model uses the laws of Thermodynamics we can run the
system in isolation and do not need to consider extraneous variables. We
don't have to consider for example the output of the sun, geothermal output or number
of SUVs on the planet but can
consider them constant for the sake of computation to see what effect a
change in a particular component plays in the overall system. We do not need to
look at tree rings or ice core data since this model uses straight forward simple mathematics
and not statistics. The starting temperature of the earth can be
obtained using the global temperature monitoring system to give us a starting
point for the equation to see if the equation is giving consistent
reasonable results. We know that if the atmosphere was nearly 100% CO2
things would be very hot indeed. So we would expect our function to
indicate that result.
The coefficients and percentage of composition for the various gases in
the air are as follows:
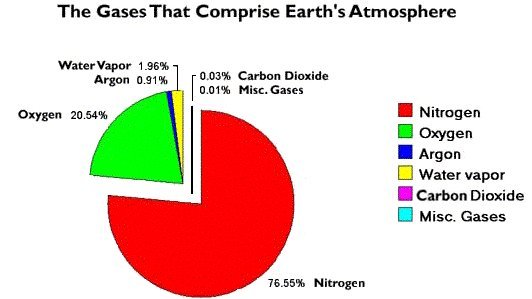
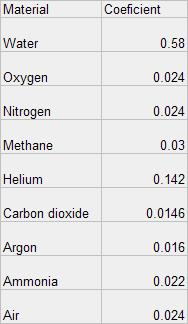
The coefficient represents the rate at which a known gas will give up itís heat.
The smaller the coefficient is the more heat that gas retains and the larger the green house effect will be.
CO2 is the best gas for retention of heat with the smallest coefficient at 0.0146. Argon is a close second.
Using a weighted average where each gas that makes up the atmosphere
and it's coefficient are combined to get the working coefficient of
the over all system. We know for example that Air has a coefficient of
0.024. When we take each gas individually and compute the working
coefficient of the over all system we get the same coefficients as for
that of combined air or again 0.024 which was experimentally obtained.
We can now take the calculated coefficient for air and plug it into the
equation for a green house
"t=f(x)" to set the base line for the computation using the known
temperature of the earth from the global temperature system as of
March 2007 and the known amount of CO2 at the time. In March of 2007
the global temperature monitoring system reported that the average
global temperature was 57.43°F and the amount of CO2 present was
0.0389%. Using the equation and the overall coefficient for air we get
the following result. This is an indication that the Thermodynamic
function for a green house is working correctly.
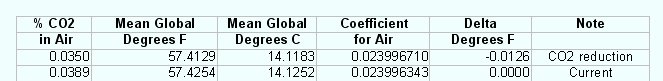
Varying the amount of CO2 in the air changes the coefficient and thus
any green house effect that it might cause. The table that follows uses
10 significant places as the change would be too small to see if less
figures were used. This gives rise to the following table were various
amounts, percentages of CO2 are introduced into the system to obtain
any change in green house effect based on a changing coefficient:
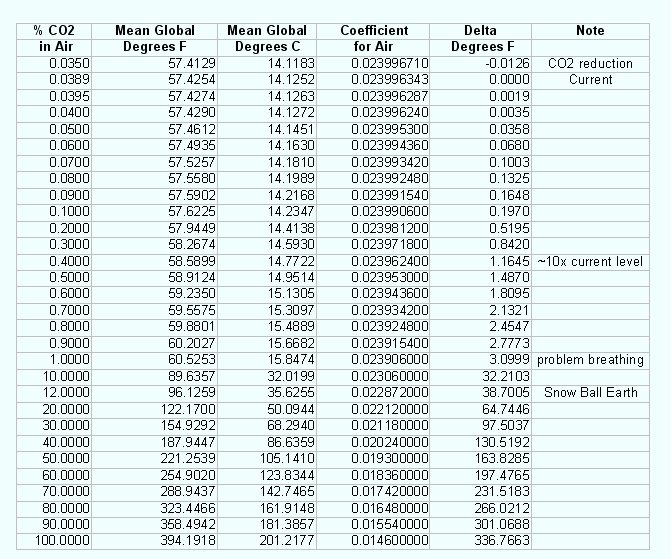
The indication is that changing a small amount of CO2 present in the
atmosphere has little or no effect on global temperature and is directly related to the overall
green house coefficient of air. In fact by the time any noticeable
change occurred from CO2 people would be dying of asphyxiation and not
higher temperatures. The chart also confirms that if the earth was at
100% CO2 the temperature would be extremely high. So there is every
reason to believe that the coefficient method accurately predicts small
changes in the effect of CO2 and any resulting temperature change.
The climate system of the earth is more complicated than this simple
model. However the model is used to only show the role that CO2 plays
in the overall Global Temperature. A rise in CO2 is absorbed by plants
for example. These plants turn CO2 into Oxygen and use the Carbon to
build the body of the plant. Reducing the amount of CO2 will make it
harder for plant life to exist and will end up killing us all. This
model is based on the hard science of coefficients and not the
scattered statistical speculation of junk science.
The overall point being is there isn't a large enough change in the coefficient to make any real difference and therefore
there will not be a large change in the retention of heat in the system and thus no substantial 'Green House' effect exists.

Doing just fine.
Bill...






